Research
Ubiquitination regulates nearly all cellular functions and is achieved by attachment of the small protein ubiquitin to a wide variety of substrates.
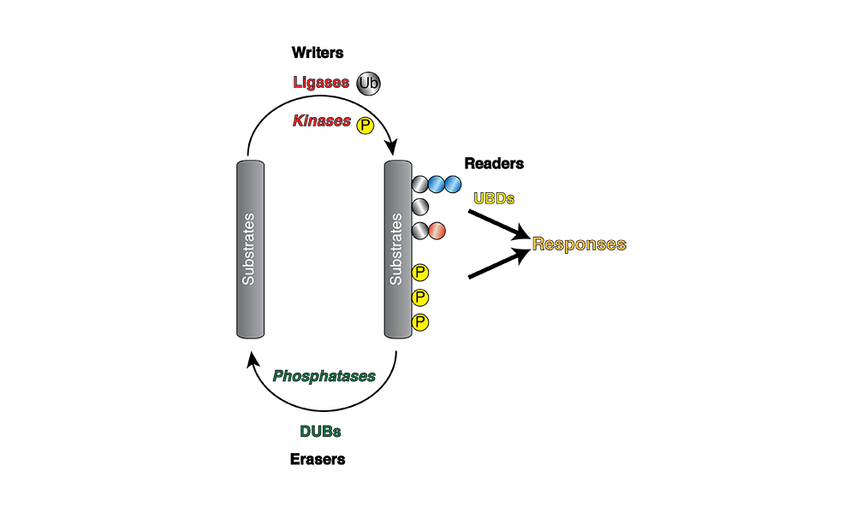
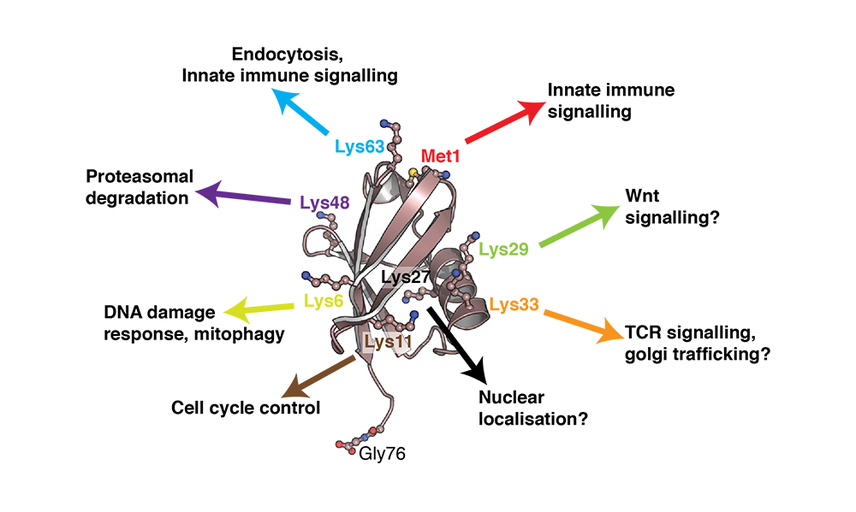
Ubiquitin regulatory versatility arises through the formation of a ubiquitin code comprised of at least eight different types of ubiquitin chains: Lys6, Lys11, Lys27, Lys29, Lys33, Lys48, Lys63 and Met1. Dedicated ‘writers’ assemble the code, ‘readers’ decipher the code and ‘erasers’ reverse the code.
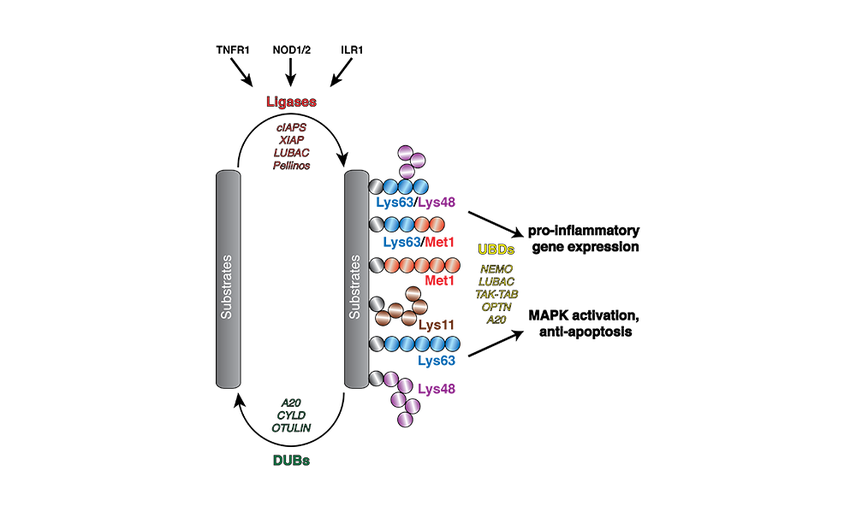
These eight ubiquitin chain types have defined cellular roles, the most characterised being Lys48-linked ubiquitin chains that serve as a signal for protein degradation. Other chain types are emerging as important regulators of cell signalling pathways. For example, Met1 ubiquitin has recently been discovered to control inflammatory signalling pathways: defects in Met1 ubiquitin chain synthesis is causative of several human auto-inflammatory and immuno-deficiency diseases.
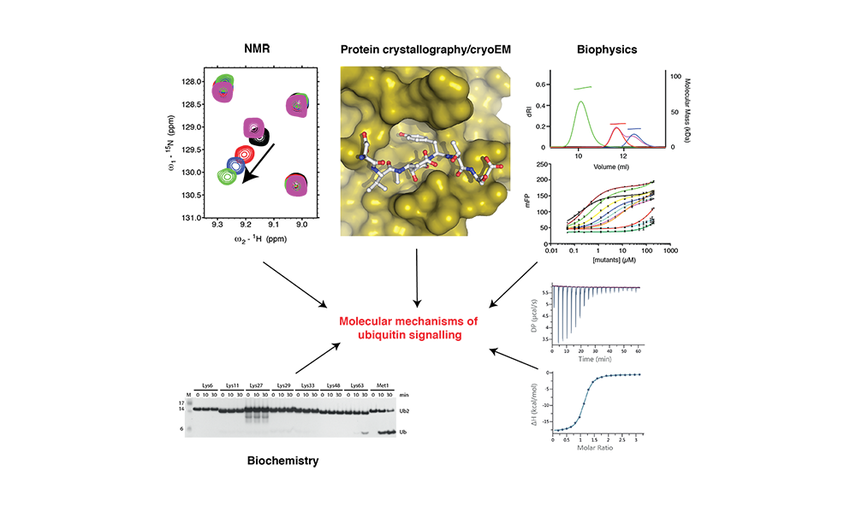
Using an integrated structural, biochemical and biophysical approach, our lab aims to understand, at the mechanistic level, how the ubiquitin code is regulated and how the ubiquitin signal controls inflammatory signalling pathways.